Frozen embryo transfer (FET) is a critical step in the in vitro fertilization (IVF) process, allowing patients to transfer previously frozen embryos into the uterus at an optimal time. For those seeking a more natural approach, natural cycle FET (NC-FET) provides an option with minimal medical intervention. This method utilizes a woman’s natural hormonal cycle to prepare the uterus for embryo implantation, reducing reliance on synthetic hormones.
This article explores natural cycle FET in depth, comparing it to medicated FET, detailing the procedure, success rates, and who may benefit most from this method.
Natural Cycle vs. Medicated Cycle FET: Key Differences
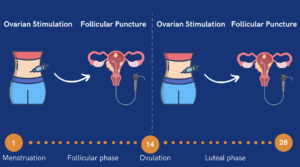
A frozen embryo transfer (FET) involves transferring embryos created in a previous IVF cycle that have been cryopreserved. This process allows individuals to attempt pregnancy without undergoing another egg retrieval cycle. Embryo transfers can be performed using either a natural cycle or a medicated cycle, depending on endometrial preparation, patient preferences, and health factors.
When undergoing FET, patients can choose between a natural cycle FET and an artificial cycle FET. In a natural cycle frozen embryo transfer (NC-FET), the embryo transfer is timed to align with a woman’s natural ovulation. Unlike a medicated FET, which uses hormone therapy to prepare the uterus, NC-FET relies on the body’s own reproductive cycle.
Understanding the differences between a natural cycle FET and a medicated FET can help determine the best approach based on cycle regularity, medical history, and personal preferences.

Medication Requirements
One of the most significant distinctions between a natural cycle FET and a medicated frozen embryo transfer cycle is the use of medication.
Natural Cycle FET relies on the body’s natural production of reproductive hormones (estrogen and progesterone) to prepare the uterine lining, with minimal or no supplemental hormone therapy.
- No ovulation-inducing drugs or hormone injections are required.
- Minimal or no progesterone supplementation may be needed post-ovulation, depending on individual progesterone levels.
Medicated FET requires estrogen and progesterone supplementation to artificially prepare the uterine lining, ensuring precise cycle control.
- Estrogen is typically administered as oral tablets, patches, or injections to stimulate the growth of the endometrial lining.
- Progesterone is introduced later to mimic the body’s natural luteal phase and support implantation.
- Ovulation is either suppressed or not needed, as the uterine environment is controlled through medication.
This means that women opting for a medicated cycle may need weeks of hormonal medication leading up to the transfer, while natural cycle patients experience a more organic hormonal environment with little to no external intervention.
Endometrial Preparation
The uterine lining (endometrium) plays a crucial role in embryo implantation. The preparation of the uterine lining is a critical part of the frozen embryo transfer process, which involves specific steps to ensure the endometrium is ready for implantation. The way it is prepared differs in natural and medicated cycles:
Natural Cycle FET:
- The endometrial lining develops naturally under the influence of the woman’s own estrogen, produced by a growing follicle in the ovary.
- As ovulation approaches, estrogen levels peak, triggering the luteinizing hormone (LH) surge and ovulation, followed by natural progesterone production from the corpus luteum to prepare the uterus for implantation.
Medicated FET:
- Estrogen is administered to build the uterine lining, which means the endometrial lining is developed artificially.
- Once the lining reaches an optimal thickness (typically 7-12mm), progesterone is introduced to mimic the body’s natural luteal phase and prepare for embryo implantation.
- Since ovulation is not required, the process can be carefully timed to match the embryo’s developmental stage.
The key difference is that in natural cycle FET, the timing depends entirely on the body’s own hormonal signals, while medicated FET allows precise control over the cycle using medication.

Monitoring & Appointments
The number of doctor visits, ultrasounds, and blood tests varies between natural and medicated cycles:
Natural Cycle FET requires more frequent monitoring to track the natural progression of ovulation.
- Blood tests and transvaginal ultrasounds monitor follicle growth, endometrial lining, and the LH surge to determine the best transfer timing.
- Embryo transfer is scheduled based on ovulation, typically 5-6 days after it occurs. This allows for less scheduling flexibility because transfer timing depends on when ovulation naturally occurs.
- Patients may need multiple visits within a short time frame (every few days) to ensure precise timing.
Medicated FET requires fewer monitoring visits because the cycle is controlled through medications. Since ovulation is not necessary, it allows for complete control over timing and predictable scheduling.
- Once the endometrial lining reaches the desired thickness under estrogen therapy, progesterone is introduced, and the scheduled frozen embryo transfer is planned accordingly.
- The ability to predict and schedule the transfer in advance makes this a more convenient option for many patients, as embryo transfer can be planned in advance and fewer monitoring appointments are needed.
In short, natural cycle FET demands more flexibility and frequent check-ups, while medicated FET is more predictable with fewer clinic visits. However, recent research indicates that variations in NC-FET protocols do not significantly impact implantation or pregnancy outcomes, providing more flexibility to both fertility doctors and patients in how they approach NC-FET.
Patient Profile & Preferences
Choosing between natural and medicated FET depends on a woman’s individual cycle regularity, hormonal balance, and personal preference.
- Natural Cycle FET is best for women who:
- Have regular menstrual cycles and ovulate predictably.
- Prefer a low-medication approach and avoid taking hormonal medications whenever possible.
- Want to minimize side effects associated with estrogen or progesterone therapy.
- Have had success with previous natural conceptions or transfers.
- Are not concerned about the unpredictability of natural ovulation timing.
- Medicated FET is better for women who:
- Have irregular or unpredictable cycles, making it difficult to track ovulation.
- Need precise scheduling due to work, travel, or other commitments.
- Have a history of ovulatory disorders (e.g., PCOS, hypothalamic dysfunction).
- Have had unsuccessful FET cycles in the past and need a more controlled approach.
- Are undergoing gestational surrogacy, where cycle coordination between the surrogate and embryo transfer is required.
The Natural Cycle FET Process
Candidate Selection
Fertility specialists carefully evaluate each patient before recommending a natural frozen embryo transfer (FET). The selection process ensures that the patient’s natural cycle can support a successful embryo implantation without the need for hormonal intervention.
- The first step in candidate selection is a detailed medical history review, where specialists assess past menstrual cycle patterns, ovulation regularity, and previous pregnancy outcomes. Patients with consistent, predictable cycles and confirmed ovulation are more likely to benefit from a natural cycle approach.
- Next, hormonal evaluations are conducted through blood tests measuring estrogen, luteinizing hormone (LH), and progesterone levels at different stages of the cycle. These tests confirm whether the body is producing the necessary hormones in the right amounts to prepare the uterine lining for implantation.
- Ultrasound monitoring is also a key component of the selection process. Specialists use transvaginal ultrasounds to check follicular development and measure endometrial thickness. A well-developed follicle and a sufficiently thick uterine lining (typically at least 7mm) indicate that the body is naturally preparing for implantation.
If any abnormalities are detected, such as irregular ovulation, inadequate hormone levels, or a thin endometrial lining, the fertility team may recommend an alternative approach, such as a medicated FET cycle to provide greater cycle control. By conducting this thorough evaluation, fertility specialists ensure that each patient receives a treatment plan optimized for their unique reproductive health needs.

Monitoring the Menstrual Cycle
Since natural cycle frozen embryo transfers (FET) depend on the body’s natural ovulation, precise monitoring is essential to ensure the embryo is transferred at the optimal time. Fertility specialists track ovulation using a combination of ultrasounds, blood tests, and at-home predictor kits.
- Transvaginal ultrasounds monitor follicular growth, endometrial thickness, and uterine conditions to confirm a receptive environment for implantation. A lining of at least 7mm is typically required for a successful transfer.
- Blood tests measure key hormone levels throughout the cycle. Rising estrogen (E2) signals follicular development, while a luteinizing hormone (LH) surge indicates ovulation is imminent. Post-ovulation progesterone levels confirm that the body is producing enough support for implantation.
- Ovulation predictor kits (OPKs) detect the LH surge, helping patients anticipate ovulation. However, since OPKs can sometimes yield false positives, specialists often rely on blood tests and ultrasounds for confirmation.
By combining these monitoring methods, fertility teams can accurately time the embryo transfer to maximize the chances of implantation and pregnancy.
Confirming Ovulation
Precise ovulation confirmation is crucial before scheduling the embryo transfer. Fertility clinics use a combination of methods to verify that ovulation has occurred and that the uterus is ready for implantation.
- LH surge detection signals that ovulation is imminent. This is typically measured through blood tests or urine-based ovulation predictor kits (OPKs).
- Progesterone blood tests are performed a day or two after ovulation to confirm that the corpus luteum is producing enough progesterone to support implantation.
- Trigger shots (hCG injections) may be administered in cases where ovulation timing is uncertain. This helps synchronize ovulation with endometrial receptivity, ensuring the best possible conditions for embryo transfer.
Once ovulation is confirmed, the embryo transfer is scheduled approximately 5-6 days later, aligning with the embryo’s developmental stage for optimal implantation success.
Timing the Embryo Transfer
The success of a natural cycle FET depends heavily on perfectly aligning the embryo transfer with the body’s implantation window.
Key Factors That Influence Transfer Timing
- Day of ovulation – The embryo transfer typically occurs 5-6 days after ovulation to match the natural implantation period.
- Endometrial thickness – A lining measurement of at least 7mm is considered ideal. If the lining is too thin, the cycle may be canceled.
- Embryo stage – A day 5 blastocyst is transferred 5 days after ovulation, while a day 6 blastocyst is transferred 6 days post-ovulation.
Some clinics use endometrial receptivity assays (ERA tests) to assess the optimal transfer window for patients who have experienced previous implantation failures.
During the transfer, the embryo is placed into the uterus using a thin catheter, and no anesthesia is required. The procedure is quick, painless, and similar to a pap smear.
Luteal Phase Support
Even though natural cycle FET relies on the body’s natural progesterone production, some patients may still need additional progesterone supplementation to improve implantation success.
Who Needs Luteal Phase Support?
✅ Women with low progesterone levels after ovulation.
✅ Patients with a history of luteal phase defects or past implantation failures.
✅ Those undergoing NC-FET with a trigger shot, as this can sometimes affect progesterone production.
Types of Progesterone Support
- Vaginal progesterone suppositories – Commonly used; directly absorbed into the uterus.
- Oral progesterone pills – Less common but an option for those who prefer oral medication.
- Intramuscular progesterone injections – Typically used in medicated FET but may be prescribed in some natural cycles.
Supplementation is typically continued until pregnancy is confirmed via blood tests (around 10-14 days post-transfer). If pregnancy occurs, progesterone support may continue into the first trimester to support early development.
What to Expect During and After the Procedure
The Day of the Transfer
On the day of the embryo transfer, patients can expect a straightforward and relatively painless procedure. The embryo transfer itself is performed using a thin catheter, which is inserted through the cervix into the uterus under ultrasound guidance. The embryo is then carefully deposited into the uterine cavity in a process that takes only a few minutes.
Most patients do not require sedation for the transfer, as it is generally well-tolerated. However, mild cramping or discomfort may occur, similar to what one might experience during a routine gynecological exam. Some clinics may offer a mild sedative or muscle relaxant for patients who feel particularly anxious about the procedure. After the transfer, patients are usually advised to rest for a short period before resuming normal activities, though strenuous exercise and heavy lifting should be avoided for a few days.
Post-Transfer Care & Luteal Phase Support
Following the embryo transfer, the luteal phase is a critical period for implantation. In a natural cycle FET, the body produces its own progesterone to support the early stages of pregnancy. However, some clinics still prescribe progesterone supplementation to help stabilize the uterine lining and improve implantation rates. This may be administered in the form of vaginal suppositories, intramuscular injections, or oral medications.
Patients may experience a range of post-transfer symptoms, including mild bloating, cramping, and spotting. These symptoms are common and do not necessarily indicate whether implantation has occurred. However, if severe pain, heavy bleeding, or fever develops, it is important to contact a fertility specialist immediately.
Pregnancy Test & Next Steps
Approximately 10 to 14 days after the embryo transfer, a blood pregnancy test (beta-hCG) is performed to determine whether implantation was successful. This test measures the level of human chorionic gonadotropin (hCG), a hormone produced by the developing embryo.
If the test is positive, follow-up blood tests may be required to monitor hCG levels and ensure the pregnancy is progressing as expected. If the test is negative, the fertility specialist will review the cycle to determine possible reasons for the unsuccessful implantation and discuss options for future attempts. While a failed cycle can be disappointing, many patients go on to have successful pregnancies with subsequent transfers.
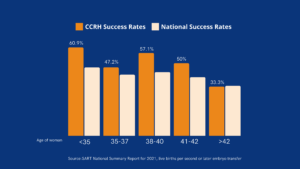
Natural vs. Medicated FET: Success Rates & Key Outcomes
Pregnancy Rates & Live Birth Rates
Many patients wonder whether natural or medicated FET offers higher success rates. Research shows that pregnancy and live birth rates for both methods are comparable, though individual success depends on factors like embryo quality, uterine receptivity, and patient-specific characteristics.
Success rates per transfer are similar in both approaches, typically ranging from 50-70% per cycle, depending on embryo quality and patient health. Likewise, live birth rates show no significant statistical differences between natural and medicated FET.
Some studies suggest medicated FET may have slightly higher implantation rates due to more controlled endometrial preparation and a reduced risk of cycle cancellation. However, natural cycle FET may lead to better pregnancy outcomes in women with regular cycles, as it avoids potential side effects from artificial hormone exposure.
Overall, both approaches are effective, and the best option depends on individual circumstances and medical history.
Potential Risks & Complications
While both natural and medicated FET are considered safe and effective, each approach has potential risks and drawbacks.
Risks of Natural Cycle FET
Cycle Cancellation Due to Ovulation Timing Issues
- In NC-FET, the exact timing of ovulation must be carefully monitored. If ovulation occurs unexpectedly early or is missed, the cycle may need to be canceled.
- Studies indicate cycle cancellation rates range from 5-20% in NC-FET, depending on the clinic’s monitoring protocols.
Limited Flexibility in Scheduling
- Since NC-FET depends on a woman’s natural ovulation, the exact date of embryo transfer cannot always be planned in advance.
- This can create logistical challenges for patients and clinics.
Potential for Luteal Phase Deficiency
- Some women may not produce enough progesterone naturally, which could affect implantation success.
- Although progesterone supplementation is less common in NC-FET, some clinics still prescribe it as a precaution.
Risks of Medicated FET
Higher Risk of Hormonal Side Effects
- Estrogen and progesterone supplementation can cause side effects like bloating, headaches, mood swings, and fatigue.
- Some women report weight gain and fluid retention due to high estrogen doses.
Increased Risk of Adverse Obstetric Outcomes
- Research suggests medicated FET cycles may be associated with slightly higher risks of pregnancy complications, such as:
- Hypertensive disorders (gestational hypertension & preeclampsia)
- Placenta-related complications (placenta previa & placenta accreta)
- Higher rates of large-for-gestational-age (LGA) babies
Potential for Over-Suppression of Natural Hormones
- Since medicated FET bypasses natural ovulation, the body does not produce its own progesterone.
- Women undergoing medicated FET must take progesterone supplements for 10-12 weeks to support pregnancy.
- If progesterone support is accidentally stopped too early, there is a risk of pregnancy loss.
Neonatal Outcomes
A major concern for fertility patients is whether the method of embryo transfer (natural vs. medicated) affects the health of the baby. Fortunately, research suggests that neonatal outcomes are similar between both methods.
Birth Weight & Growth
Studies indicate that:
- No significant difference in birth weight exists between NC-FET and medicated FET babies.
- Some studies suggest that medicated FET may be linked to a slightly higher risk of large-for-gestational-age (LGA) babies, but the difference is not always statistically significant.
- Babies born from NC-FET cycles tend to have birth weights closer to naturally conceived pregnancies.
Risk of Preterm Birth & Neonatal Complications
- Overall, preterm birth rates are comparable between natural and medicated FET cycles.
- Some research suggests medicated FET may be linked to a slightly higher risk of pregnancy-induced hypertension, which could contribute to preterm birth.
- Neonatal intensive care unit (NICU) admission rates appear similar between the two methods.
Long-Term Child Health
- Studies tracking children born via FET show no major long-term health differences between natural and medicated cycles.
- Research continues to explore whether hormonal exposure in medicated FET has any long-term impact on metabolic health, but no conclusive evidence has been found.
Recent Advances and Considerations in Natural Cycle FET
Individualized Treatment Plans
In recent years, there has been a growing trend toward individualized treatment plans in fertility medicine. Rather than following a one-size-fits-all approach, fertility specialists are increasingly tailoring natural cycle FET protocols based on factors such as cycle regularity, hormonal profiles, and endometrial receptivity. This personalized approach allows for better optimization of embryo transfer timing, leading to improved success rates.
Technological Innovations
Advancements in monitoring technologies have also contributed to the evolution of natural cycle FET. Enhanced ultrasound imaging and real-time hormone tracking have made it easier to predict ovulation with greater accuracy. Additionally, at-home ovulation detection kits that measure luteinizing hormone (LH) surges can help patients track their cycles more effectively, reducing the need for excessive clinic visits. These innovations are making natural cycle FET more accessible and convenient for a wider range of patients.
Extended Embryo Transfer Window
A recent 2024 study published in Reproductive BioMedicine Online introduced a new approach to natural cycle frozen embryo transfer (NC-FET) that could improve flexibility and success rates. The research suggests that the traditional timing of embryo transfer (typically 5 to 6 days after ovulation) could be safely extended to a 7-day window without negatively impacting implantation or pregnancy outcomes.
Is Natural Cycle FET Right for You?
Deciding whether natural cycle FET is the right approach depends on multiple factors, including past treatment experiences, embryo quality, and overall reproductive health. Women with regular menstrual cycles, normal ovulation patterns, and a history of good endometrial development may find that natural cycle FET offers a simple and effective way to achieve pregnancy without unnecessary medications.
However, for individuals with irregular cycles, hormonal imbalances, or a history of implantation failure, a medicated FET cycle may offer better control over the timing and hormonal environment needed for a successful pregnancy. The best way to determine the most suitable approach is to consult with a fertility specialist.
If you’re considering natural cycle FET as part of your fertility journey, schedule a consultation at our fertility clinic today to explore your options and develop a personalized treatment plan tailored to your needs.
Natural Cycle Frozen Embryo Transfer FAQ
What is the normal cycle for a frozen embryo transfer?
A natural cycle frozen embryo transfer (FET) follows a woman’s natural menstrual cycle, using the body’s own hormones to prepare the uterine lining for implantation. The process typically involves monitoring ovulation through blood tests and ultrasounds to determine the optimal transfer day. Once ovulation is confirmed, the embryo is transferred at the appropriate stage of development, usually 5-7 days later.
What is the natural cycle trigger of FET?
In a natural cycle FET, ovulation is the body’s natural trigger that signals when the uterine lining is ready for embryo implantation. This is usually detected through a luteinizing hormone (LH) surge or confirmed with a progesterone blood test. Some clinics may use a small dose of hCG (human chorionic gonadotropin) to ensure precise timing of ovulation.
On which day of the natural cycle is frozen embryo transfer done?
The timing of a natural cycle FET depends on when ovulation occurs. Typically, a blastocyst-stage embryo (day 5 or 6) is transferred five days after ovulation, while a cleavage-stage embryo (day 3) is transferred three days after ovulation. Ultrasound and hormone monitoring help confirm the best day for the transfer.
How do I prepare for a natural FET?
Preparation for a natural FET involves tracking ovulation using blood tests and ultrasounds to determine the right transfer window. Lifestyle factors such as maintaining a balanced diet, reducing stress, and avoiding smoking or excessive caffeine can also help optimize implantation conditions. Your doctor may recommend taking prenatal vitamins and, in some cases, progesterone supplementation after ovulation.
What is the success rate of natural cycle frozen embryo transfer?
Success rates for natural cycle FET are comparable to medicated FET, typically ranging from 30% to 50% per cycle, depending on factors such as embryo quality, uterine lining thickness, and maternal age. Some studies suggest that natural FET may have slightly higher implantation rates since the uterine lining develops in a more physiologic manner. However, individual success rates vary, and consulting a fertility specialist is the best way to understand personal chances of success.
Is medicated or natural FET better?
Both medicated and natural FET have advantages, and the best approach depends on individual circumstances. Medicated FET provides more control over cycle timing and is preferred for those with irregular cycles or ovulatory dysfunction. Natural FET, on the other hand, avoids hormone medications and may be a better option for those with regular ovulation and a history of good endometrial response.
Is natural cycle FET cheaper?
Yes, natural cycle FET is often less expensive than medicated FET because it requires fewer medications and fewer clinic visits for hormone monitoring. However, the cost difference varies by clinic and location. While natural FET may lower medication expenses, additional monitoring and potential cycle cancellations can impact the overall cost-effectiveness of the approach.