Frozen Embryo Transfer in California
A frozen embryo transfer (FET) is the transfer of an embryo which has been previously frozen, and subsequently thawed, into the uterus. Traditionally, IVF has involved ovarian stimulation followed by egg retrieval and fertilization of harvested eggs, followed by a fresh embryo transfer (ET) of an embryo into the uterus within 5 days of the egg retrieval procedure, also known as IVF-ET. With the advent of advanced embryo freezing and thawing techniques achieving extremely high embryo survival rates, traditional IVF-ET (using fresh embryos) has become less common, giving way to the more commonly practiced FET.
Frozen Embryo Transfer
Frozen embryo transfer (FET) cycles have become essential components of the IVF process and therefore must be performed with great care to achieve a successful outcome. Several components make up a successful FET cycle. A proper evaluation of the uterine cavity to rule out the presence of an intracavitary lesion (such as a polyp or fibroid that may interfere with implantation) must be undertaken prior to the FET cycle. The majority of FET cycles are medicated FET cycles, where estrogen supplementation is first administered in order to build up the uterine lining (known as the endometrial echo complex under ultrasound evaluation), until an optimal thickness of the lining is achieved. This phase of the FET cycle is critical and the type of and method of estrogen supplementation used (oral estrogen pills, vaginal estrogen suppositories, injectable estrogen, subcutaneous estrogen), the dose of estrogen, and the length of time of estrogen supplementation are critical and must be customized and adjusted to each patient based on multiple factors, so that a receptive uterine lining is achieved. The second phase of a medicated FET cycle involves progesterone supplementation, introduced to support the lining, once an optimal uterine lining has been achieved. In medicated FET cycles, progesterone is introduced while the estrogen supplementation is adjusted and continued. As in the case of estrogen supplementation, the type, dose, and route of progesterone supplementation, is critical. Commonly, progesterone is introduced in the form of intramuscular daily injections five days prior to the embryo transfer of a frozen-thawed embryo. Progesterone can also be administered in the form of vaginal suppositories or a combination of intramuscular injections and vaginal suppositories. The frozen embryo transfer must timed accurately to the initiation of progesterone supplementation in order for the FET to be successful. Estrogen and progesterone supplementation is normally continued after the embryo transfer and through 10 weeks of gestation.
An unmedicated FET cycle, also known as a natural cycle FET, is normally performed without any estrogen or progesterone supplementation. Instead, the estrogen produced by a naturally growing ovarian follicle, followed by progesterone produced after spontaneous ovulation of that follicle; support the implantation of a frozen-thawed embryo, when the FET is timed properly to the time of ovulation. Natural cycle FETs do not allow for flexibility in the timing of the FET and are only appropriate for patients with normal menstrual cycles, where ovulation is easy to monitor and is predictable.
In certain clinical scenarios, a stimulated FET cycle is performed. In a stimulated FET cycle the patient administers gonadotropin hormone injections (or oral ovulation induction medications) to induce the growth of a follicle or follicles. The growth of follicles leads to the endogenous production of estrogen which then leads to the thickening of the uterine lining. Once follicles reach a mature size, they are triggered to ovulate, leading to the production of endogenous progesterone, which then sets the stage for the embryo transfer of a frozen-thawed embryo. Stimulated FET cycles may be used in patients who do not ovulate naturally or in cases where traditional medicated FET cycles have failed.
Frozen embryo transfer cycles allow for great flexibility in optimization of the uterine lining prior to thawing of embryos, so that embryos are not thawed until the uterine lining is receptive. The essential contributor needed to achieve an optimally thick and receptive uterine lining, is estrogen. In cases of an inadequate uterine lining during an FET cycle, in addition to variations in the type of estrogen medication, dose, and route of administration, several other supplements can be added to optimize the lining thickness (including baby aspirin, pentoxifylline, vitamin E, Viagra, G-CSF…).
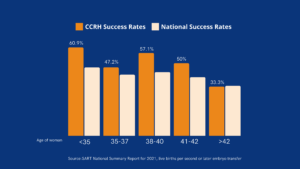
CCRH IVF Success Rates
The California Center for Reproductive Health has consistently delivered high IVF success rates, exceeding national averages in practically all age groups. Cumulative live birth rates for 2021 (the latest reporting year) are featured below:
Don’t just take our word for it!
Listen to what our patients have to say.
3,000+

20+

2X
FAQ
Reproductive endocrinology and Infertility is a sub-specialty of Obstetrics and Gynecology. In addition to managing medical and surgical treatment of disorders of the female reproductive tract, reproductive endocrinologist and infertility (REI) specialists undergo additional years of training to provide fertility treatments using assisted reproductive technology (ART) such as in vitro fertilization.
Reproductive endocrinologists receive board certification by the American Board of Obstetrics and Gynecology in both Obstetrics and Gynecology and Reproductive Endocrinology and Infertility.
In general, patients should consider consulting with an REI specialist after one year of trying unsuccessfully to achieve pregnancy. The chance of conceiving every month is around 20%, therefore after a full year of trying approximately 15% of couples will still not have achieved a pregnancy.
However, if a woman is over the age of 35 it would be reasonable to see a fertility specialist earlier, typically after 6 months of trying.
Other candidates to seek earlier treatment are women who have irregular menses, endometriosis, fibroids, polycystic ovary syndrome (PCOS), women who have had 2 or more miscarriages, or problems with the fallopian tubes (prior ectopic pregnancy).
Approximately 1/3 of the time cause for infertility is a female factor, 1/3 of the time a male factor, and the remaining 1/3 a couples’ factor.
At CCRH, we emphasize the importance of establishing a correct diagnosis. Both partners undergo a comprehensive evaluation including a medical history and physical exam.
Furthremore, the woman’s ovarian reserve is assessed with a pelvic ultrasound and a hormonal profile. A hysterosalpingogram (HSG) will confirm fallopian tube patency and the uterine cavity is free of intracavitary lesions. A semen analysis is also obtained to evaluate for concentration, motility, and morphology of the sperm.
Additional work up is then individualized to direct the best possible treatment option for each couple.
In vitro fertilization (IVF) is the process that involves fertilization of an egg outside of a woman’s body.
The process starts with fertility drugs prescribed to help stimulate egg development. In your natural cycle, your body is only able to grow one dominant egg, but with stimulation medication we can recruit multiple eggs to continue to grow. After about 8-10 days of stimulation, the eggs are surgically retrieved and then fertilized with sperm in a specialized laboratory. Fertilized eggs are then cultured under a strictly controlled environment within specialized incubators in the IVF laboratory for 3-5 days while they develop as embryos. Finally, embryos (or an embryo) are transferred into the uterine cavity for implantation.
Before deciding if IVF is the right choice, it’s important to sit down with an REI specialist to discuss available treatment options. For some people, other methods such as fertility drugs, intrauterine insemination (IUI) may be the best first choice treatment. At CCRH, we believe each individual couple is unique and not everyone needs IVF.
While not painful, the fertility medications may some side effects including headaches, hot flashes, mood swings, and bloating. The injection sites may also bruise.
Unfortunately, no. Many people think once they start IVF it’s a matter of time that they will be pregnant and have a baby. But according to national statistics per the Society of Assisted Reproduction (SART), on average 40% of assisted reproduction cycles achieve live births in women under age 35. The chances of success then continue to decrease with advancing age.
At CCRH, we employ only evidence-based interventions to ensure patient safety and optimal outcome. While we cannot guarantee a baby, we guarantee that you will receive the best, most advanced, personalized care to help you maximize your chance of a baby.
The average IVF success rate (success measured in live birth rate) using one’s own eggs begins to drop around age 35 and then rapidly after age 40. This is due to the decline in egg quantity and egg quality as a woman ages.
Our clinic’s success rate consistently beats the national average year after year.
Individual insurance plans often do not have any coverage for infertility treatments. If you have a group plan, you can call members services to see if they have coverage for infertility (including consultation/workup and IVF).
After your consultation with our REI specialist, one of our dedicated account managers with sit with you to go over the cost of treatment.