PGT Specialist
Preimplantation genetic testing (PGT) is a laboratory technique that permits chromosomal and genetic analyses of embryos prior to embryo transfer. This allows for embryo transfer of only those embryos which are free of specific chromosomal abnormalities or genetic disorders. Couples with a history of recurrent pregnancy loss due to a chromosomal abnormality, or a family history of specific genetic diseases, who are found to be carriers of the defective chromosomes or genes, can have PGT performed on their embryos in order to avoid transfer of affected embryos. The indications of PGT have expanded to women of advanced reproductive age, and family balancing as well (gender selection).
PREIMPLANTATION GENETIC TESTING (PGT)
Prior to the advent of PGT, couples who were at risk of having a chromosomally abnormal child (women of advanced reproductive age, carriers of a chromosomal abnormality), or those who were carriers of a specific genetic mutation, had to resort to prenatal testing in the first trimester (chorionic villus sampling = CVS), or in the second trimester (amniocentesis), to determine whether their fetus was affected with the disease. This meant that a definitive diagnosis would not be obtained prior to 11-13 weeks gestation (in the case of CVS), or 16-18 weeks gestation (in the case of amniocentesis) before the couple had the option of terminating an affected fetus. Moreover, many couples who consider such a therapeutic abortion unacceptable would have no option but to continue carrying the pregnancy and thus deliver an affected child. The anxiety associated with not knowing whether the fetus is affected, along with the devastation of having to terminate or deliver an affected child, is immense. Preimplantation genetic testing can give couples the reassurance that they would not have to face such anxiety as they plan their next pregnancy.
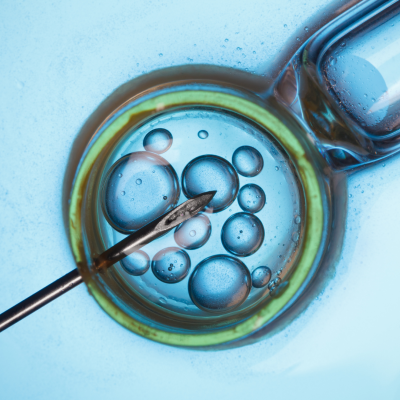
In order for PGT to be performed, couples must undergo IVF to allow for the formation of embryos in the laboratory. When embryos are usually five or six days old, a biopsy is performed by inserting a small needle into each embryo and removing several cells (called trophectoderm cells). Various techniques of gene amplification and chromosomal analysis are next applied in order to analyze removed cells for their chromosomal and/or genetic make-up. Typically, each biopsied embryo is immediately frozen after the biopsy, awaiting the results of the laboratory analysis. Embryos determined to be abnormal are discarded and normal embryos are then made available for a future frozen embryo transfer (FET) cycle.
On occasion, embryos may be maintained in culture after the biopsy (not frozen) and then normal embryos may be transferred into the uterus the very next day after a rapid-turnaround of PGT results is obtained. The safety of PGT has been documented in numerous animal and human studies.
Preimplantation Genetic Testing for Aneuploidy Screening (PGT-A)
The nucleus of every cell in the human body contains 23 pairs of chromosomes, or 46 total chromosomes. Each set of 23 chromosomes is inherited from each parent (23 from the father and 23 from the mother). When an aberrant number of chromosomes exists in a fetus, less than or greater than 46 chromosomes, aneuploidy exists. Down syndrome is an example of aneuploidy, where an extra chromosome 21 was inherited from one of the parents (typically the mom). The consequences of aneuploidy are often serious, with most aneuploid embryos failing to implant or miscarrying. Occasionally, as in the case of Down syndrome, an embryo may develop and deliver at term, with significant mental and physical impairment. Preimplantation genetic testing for aneuploidy screening (PGT-A) is a laboratory technique that permits chromosomal analysis of embryos prior to embryo transfer. Commonly, PGT-A involves comprehensive chromosomal screening (CCS), whereby all 24 chromosomes (23 chromosomes and the Y chromosome) are tested.
The most common cause for miscarriages is aneuploidy, and the most common cause for aneuploidy is advanced maternal age. As such, in couples with prior miscarriages and in women with advanced reproductive age, PGT-A has been employed prior to embryo transfer to reduce the likelihood of miscarriage. PGT-A has also been employed with the theoretic advantage of improving implantation and pregnancy rates following IVF and reducing the likelihood of delivery of an anomalous child (such as a Down syndrome-affected child).
In women of advanced reproductive age, the biggest benefit of PGT-A may be the reduction in the risk of pregnancy loss and therefore the reduction in time to the next treatment. If a patient’s embryos are determined to all be chromosomally abnormal (aneuploid) no embryo transfer is performed and the patient may begin another fertility treatment without delay. However, if embryos are transferred into the uterus without PGT-A (no chromosomal testing), and the patient conceives but later experiences a miscarriage or is diagnosed with a chromosomally abnormal fetus on first trimester testing requiring a therapeutic abortion, the patient may experience a delay of several months before fertility treatments may be initiated. Beyond the delay, the psychological and physical trauma of a miscarriage or abortion may be significant, and is one that most patients would want to avoid.
PGT-A has been conclusively proven to be useful for decreasing the likelihood of miscarriage in couples with habitual abortions determined to be due to a specific chromosomal abnormality in one of the parents, such as a Robertsonian or reciprocal chromosomal translocation. When a translocation exists in one of the parents, two different chromosomes are attached to one another. Despite the fact that the parent carrying the translocation is completely normal, this abnormality leads to the production of gametes (eggs or sperm), which are often chromosomally abnormal (missing a particular chromosome or having an extra chromosome). This often results in a chromosomally abnormal fetus and/or recurrent miscarriages. In PGT-A, chromosomally abnormal embryos are excluded from the transfer, leading to a dramatic reduction in the risk for a chromosomal abnormality in the fetus and in miscarriage risk.
The California Center for Reproductive Health is pleased to offer PGT-A to couples in need. Only safe and proven biopsy techniques are employed to ensure that embryos remain unharmed. Analysis of the chromosomal make-up of each biopsied embryo is performed by expert embryologists with outmost precision and accuracy to ensure effective preimplantation screening.
Preimplantation Genetic Testing for Monogenic Disorders (PGT-M)
Every chromosome contains thousands of different genes, which code for the human phenotype. Mutations in some of these genes may lead to specific genetic disorders. In many cases, such mutations are well defined and may be tested for. Preimplantation genetic testing for monogenic disorders (PGT-M) is a laboratory technique that permits genetic analysis of embryos prior to embryo transfer. This allows for embryo transfer of only those embryos which are free of specific genetic mutations. Couples with a family history of a specific genetic disease, who are found to be carriers of the defective genes, can have PGT-M performed on their embryos in order to avoid transfer of affected embryos.
PGT-M Indications:
- Autosomal Recessive Disorders
PGT-M may be performed for detection of specific autosomal recessive disorders. If a man and woman are found to be carriers of an autosomal recessive genetic disorder (Cystic Fibrosis, Tay-Sachs, Thalassemia, Gaucher’s…), their offspring has a 25% risk of being affected by the disease. This means that out of every four embryos created with in vitro fertilization (IVF), one embryo will be affected with the disease and three embryos will be unaffected. PGT-M would allow identification of the unaffected embryos so that they may be transferred into the uterus safely.
- Autosomal Dominant Disorders
PGT-M may also be performed for autosomal dominant genetic diseases (Achondroplasia, Huntington’s Chorea, Adult Polycystic Kidney Disease…). In such diseases, one parent is typically affected with the disorder and has a 50% chance of transmitting the disorder to their offspring. This means that out of every four embryos created with IVF, two embryos will be affected with the disease and two embryos will be unaffected. Again, PGT-M would allow identification of the unaffected embryos for transfer.
- Sex-Linked Disorders
PGT-M is further performed for the detection of sex-linked genetic disorders (Duchenne Muscular Dystrophy, Hemophilia…). In such diseases, one of the parents is a carrier of a specific mutation on one of their sex chromosomes (typically the X chromosome). In the case of an X-linked disease, if the female partner is a carrier, there is a 50% chance that if the couple has a male offspring, the boy will be affected with the disease (50% of males are affected). Female offspring have a 50% chance of being carriers, however, they typically do not manifest the disease. Therefore, if the disorder of concern is an X-linked disease, PGT-A may be employed to determine the gender of each embryo conceived with IVF (out of every 4 embryos conceived, two will be male and two will be female). Then, couples have the option of transferring only female embryos, which are not affected by the disease. If gender selection is not desired, PGT-M can be performed to determine if an embryo is affected with the disease, and transfer of that embryo can be avoided.
Virtually every genetic disease can be tested for and diagnosed with PGT-M. The California Center for Reproductive Health is proud to offer PGT-M to couples in need.
Don’t just take our word for it!
Listen to what our patients have to say.
3,000+

20+

2X
FAQ
Reproductive endocrinology and Infertility is a sub-specialty of Obstetrics and Gynecology. In addition to managing medical and surgical treatment of disorders of the female reproductive tract, reproductive endocrinologist and infertility (REI) specialists undergo additional years of training to provide fertility treatments using assisted reproductive technology (ART) such as in vitro fertilization.
Reproductive endocrinologists receive board certification by the American Board of Obstetrics and Gynecology in both Obstetrics and Gynecology and Reproductive Endocrinology and Infertility.
In general, patients should consider consulting with an REI specialist after one year of trying unsuccessfully to achieve pregnancy. The chance of conceiving every month is around 20%, therefore after a full year of trying approximately 15% of couples will still not have achieved a pregnancy.
However, if a woman is over the age of 35 it would be reasonable to see a fertility specialist earlier, typically after 6 months of trying.
Other candidates to seek earlier treatment are women who have irregular menses, endometriosis, fibroids, polycystic ovary syndrome (PCOS), women who have had 2 or more miscarriages, or problems with the fallopian tubes (prior ectopic pregnancy).
Approximately 1/3 of the time cause for infertility is a female factor, 1/3 of the time a male factor, and the remaining 1/3 a couples’ factor.
At CCRH, we emphasize the importance of establishing a correct diagnosis. Both partners undergo a comprehensive evaluation including a medical history and physical exam.
Furthremore, the woman’s ovarian reserve is assessed with a pelvic ultrasound and a hormonal profile. A hysterosalpingogram (HSG) will confirm fallopian tube patency and the uterine cavity is free of intracavitary lesions. A semen analysis is also obtained to evaluate for concentration, motility, and morphology of the sperm.
Additional work up is then individualized to direct the best possible treatment option for each couple.
In vitro fertilization (IVF) is the process that involves fertilization of an egg outside of a woman’s body.
The process starts with fertility drugs prescribed to help stimulate egg development. In your natural cycle, your body is only able to grow one dominant egg, but with stimulation medication we can recruit multiple eggs to continue to grow. After about 8-10 days of stimulation, the eggs are surgically retrieved and then fertilized with sperm in a specialized laboratory. Fertilized eggs are then cultured under a strictly controlled environment within specialized incubators in the IVF laboratory for 3-5 days while they develop as embryos. Finally, embryos (or an embryo) are transferred into the uterine cavity for implantation.
Before deciding if IVF is the right choice, it’s important to sit down with an REI specialist to discuss available treatment options. For some people, other methods such as fertility drugs, intrauterine insemination (IUI) may be the best first choice treatment. At CCRH, we believe each individual couple is unique and not everyone needs IVF.
While not painful, the fertility medications may some side effects including headaches, hot flashes, mood swings, and bloating. The injection sites may also bruise.
Unfortunately, no. Many people think once they start IVF it’s a matter of time that they will be pregnant and have a baby. But according to national statistics per the Society of Assisted Reproduction (SART), on average 40% of assisted reproduction cycles achieve live births in women under age 35. The chances of success then continue to decrease with advancing age.
At CCRH, we employ only evidence-based interventions to ensure patient safety and optimal outcome. While we cannot guarantee a baby, we guarantee that you will receive the best, most advanced, personalized care to help you maximize your chance of a baby.
The average IVF success rate (success measured in live birth rate) using one’s own eggs begins to drop around age 35 and then rapidly after age 40. This is due to the decline in egg quantity and egg quality as a woman ages.
Our clinic’s success rate consistently beats the national average year after year.
Individual insurance plans often do not have any coverage for infertility treatments. If you have a group plan, you can call members services to see if they have coverage for infertility (including consultation/workup and IVF).
After your consultation with our REI specialist, one of our dedicated account managers with sit with you to go over the cost of treatment.